Pure Protein has pioneered an approach to produce soluble forms of HLA Class I and II proteins (sHLA) for research, diagnostic, and therapeutic applications.
Soluble HLA Technology
Soluble HLA Description
Class I and Class II Human Leukocyte Antigens (HLA), are multimeric molecules to be utilized in many diagnostic and therapeutic applications which demand structural integrity and proper functionality in order to provide highest data quality in a clinically relevant manner. Naturally, HLA molecules are glycoproteins where a transmembrane domain tethers the molecule to the cell surface which considers a major obstacle for large-scale production and obtaining a product with high degree of purity. Because Pure Protein’s sHLA molecules have been truncated, lacking such transmembrane portion, the sHLA proteins are not retained on the cell surface but readily secreted into the cell culture media where they can be readily and economically separated from the producing cells.
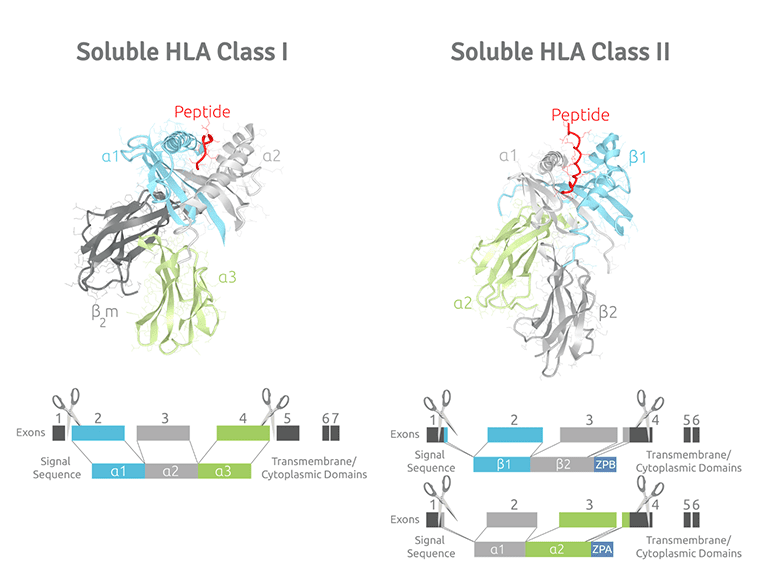
Soluble HLA Class I molecules are glycoproteins consisting of a heavy chain (comprised of α1, α2, and α3 domains), a light chain (β2m) and the peptide they present forming a trimeric complex. This trimeric protein complex is approximately 47kDa in size with a 33kDa heavy chain, an 11kDa light chain, and a 1 kDa peptide (8 to 11mer) located in the molecule's groove. In addition, various sHLA alleles for Class I have an added purification tag (VLDL) at the carboxy end of the α3 domain.
Soluble HLA Class II molecules are glycoproteins as well, however, they have an α and β chain where the α1 and β1 regions of the chains come together to make a membrane-distal peptide-binding domain, while the α2 and β2 regions, the remaining extracellular parts of the chains, form a membrane-proximal immunoglobulin-like domain. The Class II trimeric protein complex is approximately 61kDa in size consisting of a 27 kDa beta chain and a 26 kDa alpha chain with a 2 kDa peptide (15 to 24mer) in the groove. Finally, it is important to note that Class II molecules have a Leucine zipper tail at the carboxy end of the α2 and β2 region to increase stability and support assembly of the molecule within the cell.
Soluble HLA Characteristics
The foundation of Pure Protein’s sHLA protein technology centers on the quality of the products and services we offer. All soluble HLA proteins are well characterized, proven to present native, antigenic epitopes recognized by specific antibodies, demonstrate high stability and integrity of their structures and are most compliable to most application platforms as well as highly adaptable to individual needs. Forced degradation studies, executed by exposure of sHLA Class I molecules to elevated temperatures indicate that these molecules have a high tolerance for heat exposure, maintaining stability without disintegration up to 42˚C. Similar results are achieved with sHLA Class II proteins which show an immense heat tolerability of up to 50˚C. High heat stability results in better assay performance, lower amounts of protein required, the ability to work at ambient temperatures, and an extended shelf life. In addition to physical interrogations, Pure Protein has tested a plethora of specific HLA antibodies, all capable of positively recognizing public and private epitopes.
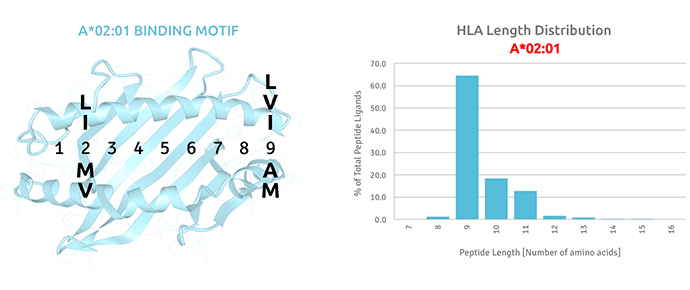
As a result of the high purity of the Pure Protein’s HLA proteins, protein sequences can be verified by using a combination of tryptic digests and mass-spectrometry analysis. Moreover, Pure Protein has performed detailed mass-spec analysis on all HLA Class I molecules and provides customers with accurate binding motifs and overall peptide length distribution, to assist customers with multivariate comparisons across alleles as well as to confirm the identical nature of sHLA compared to its native cell-bound counterpart.
Soluble HLA Coverage
HLA genes are highly polymorphic, which means that the HLA loci represents the genetically most variable gene region encoding thousands of different alleles, and thus allowing it to fine-tune the adaptive immune system. Pure Protein has developed the world’s largest selection of soluble HLA proteins and included alleles across most population and transplant patient frequencies, broad ethnicity coverage, and haplotype information.
Since HLA Class II DQ and DP molecules function as dimers containing two protein subunits, the alpha chain and beta chain, four different combinations are possible, as each individual receives one set from the maternal chromosome and the other set from the paternal chromosome making such a person a double heterozygote. Naturally, that dramatically increases the number of possible protein combinations in each individual. To offer our customers a more satisfactory solution to solve this complexity, Pure Protein formulated combinatorial matrices consisting of a selection of specific alpha and beta chains with the goal to provide combinations where each, alpha or beta chain are represented at least twice. However, there are factors that limit this variation. Some combinations seem not to be made. Furthermore, haplotypes are naturally observed where a set of variations tend to be inherited or linked together, which further reduces the combinatoric possibilities. As of 2020, Pure Protein has over 270 established cell lines in inventory from which HLA proteins are produced from.
HLA Nomenclature
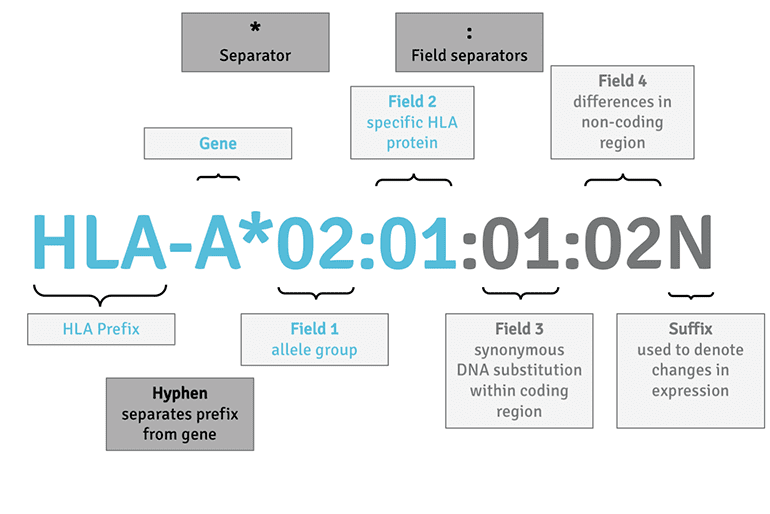
Because the genes encoding the HLA molecules are highly polymorphic, they require a systematic nomenclature. Each HLA allele name has a unique number corresponding to up to four sets of digits separated by colons. The length of the allele designation is dependent on the sequence of the allele and that of its nearest relative. Our sHLA alleles all receive a four-digit name, which corresponds to the first two fields. For researchers in the transplant field, it is important to note that the digits before the first colon describe the type, and often corresponds to the serological antigen carried by an allotype. Alleles whose numbers differ in the two fields naturally differ in one or more nucleotide substitutions that change the amino acid sequence of the encoded protein.
HLA Frequency
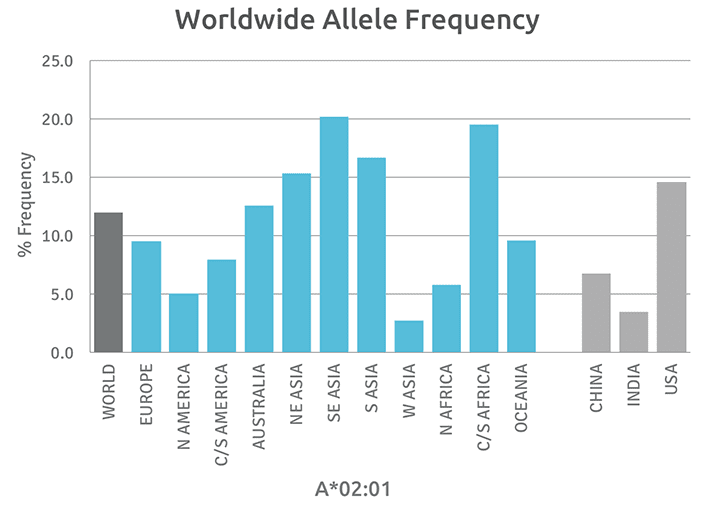
Although the number of individual HLA alleles that have been identified is large, approximately 40% of these alleles appear to be unique, having only been identified in single individuals while roughly a third of alleles have been reported more than five times in unrelated individuals. Because of this variation in the rate at which individual HLA alleles are detected, attempts have been made to categorize alleles at each expressed HLA locus in terms of their prevalence. The result is a catalog of common and well-documented HLA alleles, as well as a catalogue of rare and very rare HLA alleles. Rare alleles are defined as those that have been reported one to four times, and very rare alleles as those reported only once. We are displaying these frequencies varying by geographic region. Data were obtained from the Allele frequency net database 2020. http://www.allelefrequencies.net/hla6006a.asp
HLA Epitopes/Eplets
HLA antigens have multiple epitopes that can be recognized by specific antibodies. The original description of the epitope repertoire was based on serological cross-reactivity between HLA antigens and antibody specificities against so-called private and public determinants. Elucidation of the three-dimensional molecular structures and amino acid sequence differences between HLA antigens has made it possible to define, to a certain extent, the structural basis of HLA epitopes. The general concept is that HLA epitopes are determined by polymorphic amino acid residues on the molecular surface. As such, HLA eplets are approximations of such epitopes and defined as strings of polymorphic sites that serve as target regions for antibody binding. Our datasheets list all possible eplet assignments for any given HLA protein. Eplet information was obtained from the HLA epitope registry. https://www.epregistry.com.br/
HLA Transfectant Cell Lines
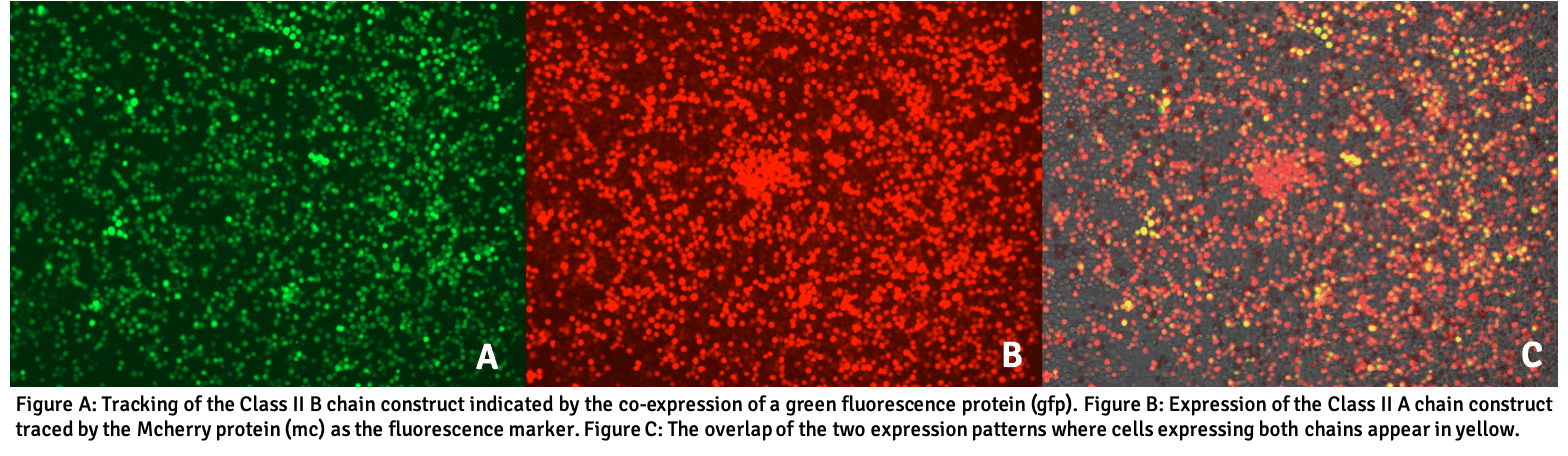
Using a proprietary transfection system, Pure Protein has established a transfection pipeline to generate new sHLA secreting molecules for all subtypes to create clinically representative panels of Class I A, B, C and Class II DR, DQ and DP alleles. Class I alleles are made with a single round of transfection by using the cells capability to self-produce the b2m light chain and form the trimeric complex. Class II generation is more challenging to generate stable cell lines. As a result, the alpha (A) chain is transfected first before adding the beta (B) chain in a second work process. The transfected cell line performs the final assembly of the HLA complex by populating the HLA groove with an endogenous peptide exactly as native HLA would be loaded. As such, all peptides are derived from the cell itself and are not encoded by the construct. Regulation of the rate at which the cells produce the sHLA protein is largely dictated by the availability of peptide ligands and chaperons that support the multistep assembly of sHLA.
Using a special color tracking system, the presence of the Class II B chain constructs can be confirmed by tracking the co-expression of a green fluorescence protein (gfp), whereas the A chain expression is monitored through a Mcherry protein (mc) as the fluorescence tracker. These colored proteins are co-produced within the transfectant cells, but are not part of the final sHLA molecule, nor are they secreted.
How can we help?
Pure Protein has developed a proprietary, novel approach for manufacturing of soluble Class I and Class II HLA proteins for research, diagnostic, and therapeutic applications.
We can help you to …
Define your own custom manufacturing needs
Do you need to:
- produce a sHLA in large quantities?
- acquire a sHLA molecule that is not listed?
- custom manufacture HLA proteins?
- utilize GMP manufactured sHLA?
Develop your own assay strategy
Do you need to:
- find suitable antibodies that recognize sHLA molecules
- couple sHLA to a solid support
- optimize your assay?
- decide on a sHLA standard?
Create your own diagnostics or therapeutics
Do you need to:
- develop a strategy that utilizes large panels of sHLA?
- acquire a commercial license?
- make your own assay platform?
- collaborate on a project?
Talk to an expert today
We understand the challenges you face. If you’re still wondering if our technology can take you to the next level and are looking for some clarity, reach out to our experts and someone from our team will get back to you in no time.
Expert advice, whenever you need it!
Featured Resources
PUBLICATION
Profiling antibodies to class II HLA in transplant patient sera.
McMurtrey C, Lowe D, Buchli R, et al.
Hum Immunol. 2014 Mar;75(3):261-70.
PUBLICATION
Large-scale production of class I bound peptides: assigning a signature to HLA-B*1501.
Prilliman K, Lindsey M, Zuo Y, Jackson KW, Zhang Y, Hildebrand W.
Immunogenetics. 1997;45(6):379-85. [PMID: 9089095]

